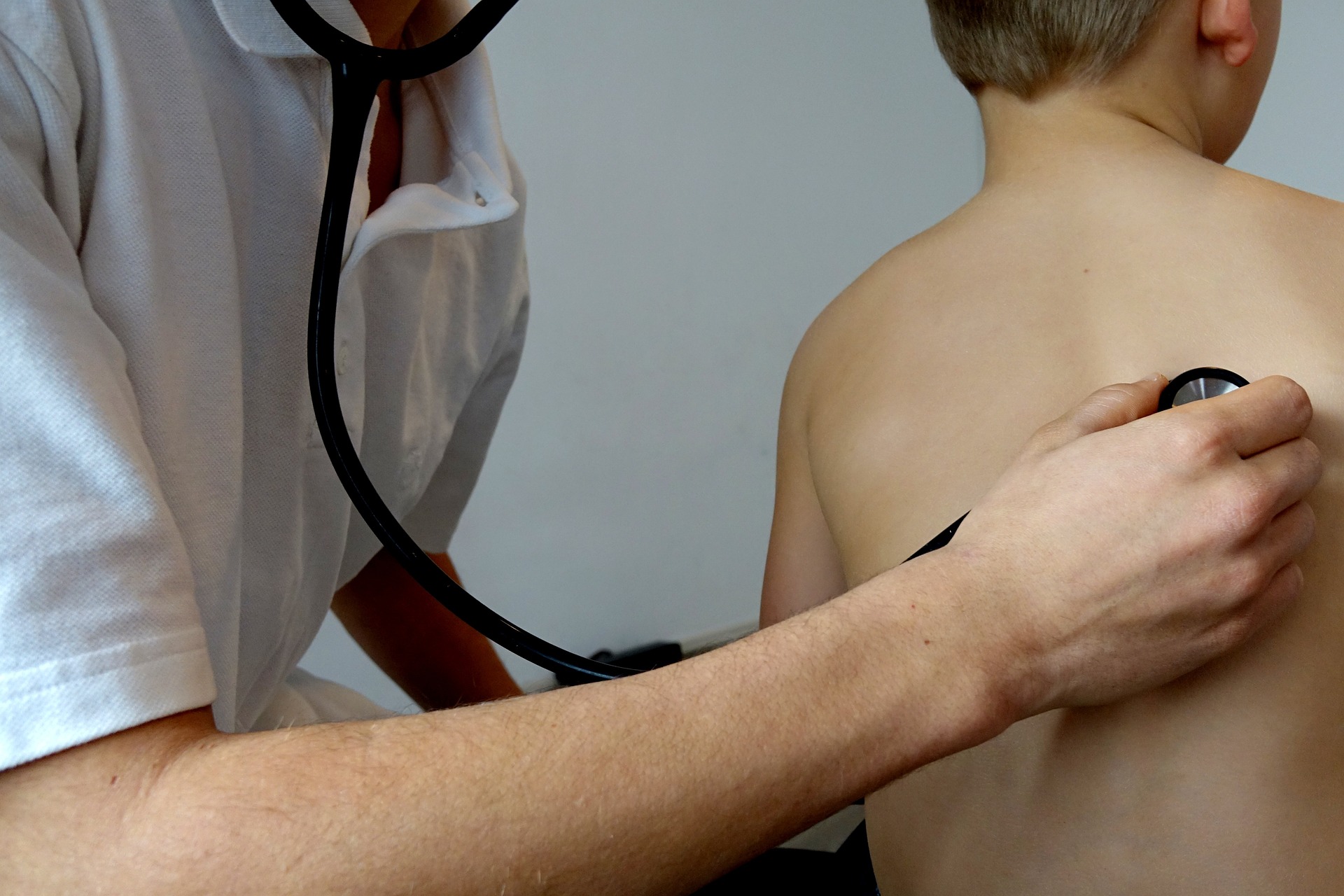
Medical Research Involving Minors
Especially since the second half of the twentieth century, medical research has led to the development of numerous therapeutic, diagnostic and preventive techniques. These advances have also been reflected in paediatrics. Yet the balance of progress in this field, compared with other areas of medicine, has been considerably more modest. In a paper from 1968 the paediatrician Harry Shirkey coined the phrase "therapeutic orphans" in order to highlight the extent to which children are neglected - in comparison to adults - when it comes to the development of new medical products and procedures.
The problem with clinical trials of drug treatments for minors
It continues to be the case that the treatment of minors with drugs frequently takes place using so-called off-label or off-licence products i.e. with substances that are either not licensed for paediatric use at all due to a lack of clinical trials or at least do not have the appropriate licence for the specific area of application. Paediatricians then have to decide on the administering of a drug based on empirical values. Most significantly, they also have to determine the dose without recourse to scientifically secure data.
Even though the off-label or off-licence use of drugs is widespread in paediatrics, this can only be a makeshift solution from the medical standpoint. Children are not "little adults", especially from the perspective of developmental physiology, which is, amongst others, highlighted by the German Academy for Pediatrics and Adolescent Medicine (DAKJ) in a statement on medical research involving minors. With reference to the international human rights conventions of the United Nations, the German academy both advocates better qualifications of physicians and nursing staff and pleads for the parliamentary representation of children’s rights in the German federal parliament ("Bundestag"). As numerous examples have demonstrated, it is therefore often not possible to simply adjust the dosage of drugs that are successfully used on adults. Cases like the “grey baby syndrome” or the off-label prescription of antidepressants to minors exemplify these controversies. A further problem consists in that the off-label use of drugs can only take place under certain conditions which were set by the Federal Social Security Court during two verdicts in 2002 and 2006 respectively, to the disadvantage of the 'Gesetzliche Krankenversicherung' (GKV) which stands for statutory health insurance.
In this context it cannot even be considered appropriate to use the blanket term "children". On the contrary, it is vital to differentiate between several developmental phases up to adulthood, each of which has its own distinct physiological characteristics. Only by conducting targeted clinical trials on minors will it be possible to really remedy the problem of children as "therapeutic orphans".
Status and regulation of clinical trials on minors
In certain areas of paediatrics, most notably in paediatric oncology, large numbers of clinical trials are already being carried out today. Indeed, estimates suggest that in the United States, for example, roughly 70% of all child cancer patients are treated as part of clinical trials. This does, however, raise the question of whether crucial distinctions between medical research and medical practice are being ignored. In Germany, too, clinical tests on minors are being carried out. According to an evaluation amongst VFA-businesses this is, however, the case stagnantly and only on a small scale.
The controversy surrounding a convincing balance between the need for clinical research on minors on the one hand and their high need for protection on the other is also reflected in the various approaches to regulating medical research on minors at international and national level. In Germany, for example, there are currently no comprehensive special statutory regulations governing research on human beings. Special laws are in place, first and foremost, for the clinical testing of pharmaceuticals (Pharmaceutical Act – AMG) and medical devices (Medizinprodukterecht-Durchführungsgesetz – MPDG [Medical Device Law Implementation Act] as a supplement to the European Medical Device Regulation – MDR).
In general, the protection of the human subject in clinical trials of pharmaceuticals is dealt with in §§ 40-42a of the AMG. It is stipulated here, inter alia, that a risk/benefit analysis must be performed prior to commencement of a clinical trial and that subjects must give their informed consent into participation after receiving relevant information. In addition, a favourable opinion is required from an independent Ethics Committee. Research on minors is discussed specifically in §§ 40 IV and 41 II. The basic principle is that clinical trials on minors are subject to additional restrictions compared to those conducted on adults: Clinical trials involving minors are permitted only if adults cannot be considered as subjects for methodological reasons, the subject of the trial promises therapeutic, diagnostic or preventive benefits for minors and, in addition, the application is medically indicated for the child subjects involved. In the case of underage subjects, consent is initially given on a proxy basis by the minor's legal representative; it must, however, comply with the presumed wish of the minor. Yet the minor, too, must be adequately informed prior to commencement of a clinical trial by a trial team member experienced in dealing with minors in a manner appropriate to their age and intellectual maturity. A refusal to participate on the part of the minor must be respected. If the minor subject themself is in a position to grasp the nature, significance and implications of the clinical trial in question (and to direct their wishes accordingly), the minor's consent must also be obtained.
The issue of the ethical acceptability of biomedical research involving minors constitutes an extraordinarily difficult problem within the ethics of research and has hence long been a subject of intense debate. The special difficulty of the problem lies in the clash between two conflicting moral intuitions: on the one hand, minors must be regarded as needing special protection, which would lend plausibility to a general ban or at least a far-reaching restriction of biomedical human experiments in the filed of paediatrics. On the other hand, the extensive restriction of paediatric research implies considerably slower medical progress in this area, if not a complete standstill, and imposes limitations on the ethical imperative that the benefits and risks of applied therapeutic methods must be reviewed by means of clinical studies.
Informed consent constitutes one of the central principles in the ethics of research on human beings. Accordingly, human experiments can only be considered ethically acceptable in principle if the test subjects in question have been advised in advance of the nature, significance and implications of an experiment and have then given their informed consent to participation. This idea is reflected in all pertinent codes of research ethics and in the in the correspondig international and national regulations. In contrast to adults, minors are frequently themselves unable to give informed consent on account of their lack of cognitive abilities. Consequently, according to one possible position, research on minors is ethically unacceptable at least in those cases when it is not associated with a direct medical benefit for the minor test subjects; in this understanding, only "therapeutic research" would be permissible. Such a position was put forward, for example, by the American bioethicist Paul Ramsey in his book "The Patient as Person" published in 1970.
That medical research on minors is necessary in order to develop new and effective techniques in paediatrics can be regarded as generally accepted. Since, however, this in no way involves a value-neutral goal, but on the contrary the provision of effective treatment options is highly desirable from the moral standpoint, many ethicists have sought arguments to counter the position of fundamental inadmissibility put forward by Ramsey and others. What is not disputed in this context is that the consent of a legal representative and the lack of alternatives are necessary conditions. Furthermore, a rough distinction can be made between four approaches which explore possible justifications for medical research on minors and possibilities for their consent.
Assumed consent
The first approach proceeds from the presumption that in light of the moral value attached to participation in a human experiment the minor's consent may be assumed. Richard McCormick elaborated this position as a direct response to Ramsey's theory of fundamental unacceptability. According to McCormick's central argument, there is absolutely no need to assume that a minor test subject would refuse to participate in a medical experiment. On the contrary, it may be assumed that the minor would give his or her consent if he/she had the requisite faculties because such a decision would be morally correct. For this reason, McCormick concludes, Ramsey's theory of the ethical unacceptability of research on minors for the benefit of others is incorrect. One readily obvious criticism of this approach is that no comparable assumption is made in the case of adults who are able to give informed consent, and hence there is no reason to accept why this should be permissible in the case of minors.
Educational benefit as a ground for justification
The point of departure for a second approach is the assertion that research involving minors can be considered ethically acceptable if the research is associated with a direct benefit for the minor test subjects. While the presumption in this context is usually a direct medical benefit, proponents of this approach claim that other types of benefit can and must also be taken into account here. A participation in a human experiment does, for instance, also have an educational benefit for minor test subjects, inasmuch as basic social skills such as altruism and solidarity are fostered. Thus, experiments that do not have a direct medical benefit can also be considered to be ethically acceptable. This appears plausible when one remembers that in many instances children are subjected to norms that are informally considered important or accepted without their being asked, since otherwise it is scarcely possible to socialize and educate children. At issue here is firstly whether an educational benefit can really acquire the asserted legitimizing force, while on the other hand many critics claim that an understanding of altruistic actions can only be assumed from a certain level of maturity onwards and hence research on younger children cannot be justified on the basis of this approach.
The concept of minimal risk
A third approach that has taken on special prominence in the US debate starts out from the risk profile of human experiments. If, so the argument goes, a human experiment entails only minimal risks and burden which in some cases can only be seen as intervention in the context of a strictly defined definition of intervention, the requirement for personal consent may be dispensed with and the consent of the legal representative suffices for ethical justification. In the first place, this approach raises the issue of whether the use of humans in medical experiments without their consent is not fundamentally problematic from an ethical perspective, irrespective of any risks and burden. If this is the case, the invoking of only minimal risks and burden cannot provide an ethical justification. What is more, this approach encounters problems arising out of the definition of risk. In particular, many contributors to the debate consider it questionable whether it is possible to define with adequate clarity what constitutes only a minimal risk and whether an objective determination lends itself to adequately accommodating the evaluations of individual test subjects. Even in regards to the judgment of group-beneficial research projects with a risk higher than minimum, the views of German ethics commissions were diverging, according to a recent study.
Empirical studies show that standards as 'minimal risk' or 'minimal stress' are interpreted differently. Therefore, these abstract distinctions become more concrete by an increasing number of clinical examples and are connected with further guidelines.
Parents as (preliminary) surrogate decision makers for their children
The medical ethicist Giovanni Maio on the other hand questions, whether research, which is not associated with the benefit of the child, indeed poses an ethically unjustifiable instrumentalization of minor test subjects. According to Maio, there is a tension between the prohibition of the instrumentalization of a test subject and the imperative of investigating possible help for children in the future. Maio claims that only the violation of the child’s dignity makes the involvement of minors illegitimate. This is why he wants to let parents decide, whether the research is appropriate.
Researchers have a duty to work closely with underage subjects and their legal guardians and to take into account their respective interests and stages of decision-making ability. The underage participant themselves should therefore be involved in the decision-making process depending on their respective mental maturity, although in practice it is often difficult to determine, whether or not a child is mature enough for the consent into a survey. If a child refuses to participate in the survey though, this decision should be respected even before the child’s maturity has been ascertained.
The distinction between medical practice and medical research
The dividing line between medical practice (diagnosis, therapy, prevention) and medical research has long proven a difficult issue. While medical practice is oriented towards the well-being of the individual patient, the goal of medical research is primarily to gain scientific insights. In many instances, however, both goals are pursued at the same time. Numerous labels are used to denote this special type of act, such as "therapeutic experiment" or experiments with a direct medical benefit for the test subjects (self-interested research). Purely scientific experiments are hence referred to as "for the benefit of others". In addition, a distinction is now made for so-called "experiments for group benefit", i.e. those that do not provide any medical benefit for the specific test subject but for the group to which the subject belongs. Membership of a group may be established in this context on the basis of a disease (e.g. Alzheimer's patients) or age (minors).
Critics assert that terms such as "therapeutic experiments" give rise to confusion between structurally different types of act - namely medical treatment, on the one hand, and scientific research, on the other. They therefore argue that all terms suggesting a third, independent type of act between medical practice and research should be done away with. Of particular importance in the ethical debate about medical research on minors is the so-called research „beneficial to group interests“.
The controversy surrounding the permissibility of group-beneficial research
As an example of the debate on the ethical permissibility of group-beneficial research on minors, the controversy surrounding the Fourth Act Amending Other Provisions of the German Drug Law of December 20, 2016 is discussed below.
Following the amendment to the law in 2016, research involving adults who lack the capacity to consent and benefits group interests can be admissible under certain conditions. In this context, it has been criticized that the subgroup of minors has not been treated equally to other subgroups of people who lack the capacity to consent. Thus, research beneficial to group interests with adults incapable of giving consent is only admissible if the experiments have a direct medical benefit for the test subjects. However, this does not apply to minors. A reason for this stipulation might be the intention to reduce off-label use of pharmaceuticals when it comes to treating minors. Furthermore, a minor’s capacity of giving consent is usually evaluated by age limits while the capacity of adults is considered on a case-by-case basis. Moreover, an existing incapacity of giving consent is taken into consideration when it comes to evaluating adults.
A key counter-argument here is that it is not comprehensible why a contingent relationship - such as the same age - between the test subjects, on the one hand, and the actual beneficiaries, on the other, should acquire an ethically legitimizing force. In particular, critics point out that one cannot automatically assume the existence of a strong group-based sense of solidarity between test subjects and research beneficiaries belonging to the same group. There is criticism concerning the 12th amendment of the German Drug Law in 2004, which legalized research that benefits a group of patients according to § 41 II Nr.2. Critics object, that the amendment contradicts the protection of the human dignity as it is determined in Art. 1 l of the German Constitution. The argument is that minors, who can’t consent to participate in an experiment and do not benefit themselves from their participation, are misused as a means to an end. Advocates of research which benefits a group of patients emphasize that the constitutional right of human dignity is not to be understood individualistically and selfishly. According to the advocates of research that benefits groups, the Federal Constitutional Court stresses that the individual is part of a society. This could also be understood as the solidarity among a community of fate, like a group of children suffering from a rare disease. Research for the benefit of a group therefore does not necessarily contradict the constitutional right of human dignity as long as the degree of the individual’s protection is sufficiently high.
Special Cases on Studies with Minors
Although the same legal and ethical standards apply for all sections of pediatrics, two priorities can be distinguished. Regarding the establishment of surveys, the pediatric oncology stands out significantly from the other fields. As a consequence of the realization of multicentric studies, new treatment options have been achieved in course of which the cure rate of minors suffering from cancer was increased from 15% in the early 1970s to round about 70% today. However, physicians and ethicists are confronted with the difficult task of informing parents and children as well as balancing the protection of test subjects and the knowledge gained by the study. The high quantity of studies furthermore raises the question, whether or not there is a disregard of significant differences between medical research and medical practice.
According to experts, many medicaments are used in the field of neonatology, which have not been tested on groups of patients before. The occasionally grave consequences for newborns leads experts to question, whether all neonatological treatments should not be carried out as part of clinical studies. However, the distinction of research and medical practice would then be up for discussion as well.